Why Does Iodine React Faster Than Bromine
Rate of SN1 reaction depends on type of alkyl group attached with halogen rate of reactions follows following order Tertiary halo alkane Secondary haloalkane Primary halo. This answer is not useful.
Which Is More Electronegative Bromine Or Iodine Quora
It is because bromine is smaller in size than iodine and hence easily reacts.
. Iron bromine ironIII bromide. When starting The Iodine Protocol or just increasing our unrefined salt intake we may find ourselves dislodging and detoxing. The reddish-brown bromine is decolourised as it reacts with the alkene.
Given that halogen reactivity is all about gaining electrons the fewer valence shells in atom the closer to the attractive nucleus the outer valence is and so the more violently attracted to the. A liquid alkene like cyclohexene can be shaken with liquid bromine or its solution in tetrachloromethane. Show activity on this post.
Since bromine has fewer shells its outer shell is closer to the nucleus so. The reaction is faster than that of iodine but slower than that of chlorine. It is not the leaving group ability of ceF- which makes the reaction go faster than with say bromine or chlorine but its very high negative inductive effect due to its large.
See answer 1 Best Answer. But usually halogen gases tendency to undergo reduction to become halide. Chlorine is highly reactive and thus reacts faster than bromine.
For bromine or iodine to react each atom needs to gain an electron to fill up its shell so that it is in a more stable state. Bromine - Why Iodine can change the world. Bromine has a less vigorous reaction.
Since bromine has less number of shells the outer shell is closer to the nucleus and. Why is iodine less reactive than bromine. It is because bromine is smaller in size than iodine and hence easily reacts.
Another reason is that bromine is the more electronegative. Fluorine is even more reactive too much and this is why we dont see it in free radical halogenation. Iodine has 5 shells whereas bromine has 4 shells.
Another reason is that bromine is the more electronegative than iodine and hence it attracts. All four of the ions are reducing agents.
Why Is The Reactivity Of Iodine Lower Than That Of Bromine Quora
Solved 5 Iodine Is A Better Leaving Group Than Bromine Chegg Com
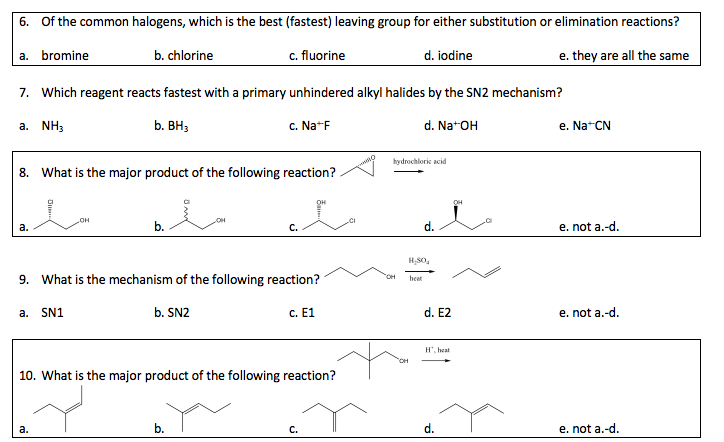
Solved 6 Of The Common Halogens Which Is The Best Chegg Com
Which Would Undergo An Sn2 Reaction Faster Bromoethane And Chloroethane And Why Quora
No comments for "Why Does Iodine React Faster Than Bromine"
Post a Comment